- Home Page
- Company Profile
-
Our Products
- Pharmaceutical Injection
- yervoy 50 mg 10 ml(ipillimumab)
- Cefuroxime 250mg(Restum)
- Mitomycin Injection
- etanercept solution for injection 25mg
- Denosumab 60mg/mL (r-DNA origin) in Pre-Filled Syringe
- Mucosot Acetylcysteine Injection
- Eptifab 20mg/10ml Vial
- VASOPRESSIN INJECTION IP 20 UNITS /ML
- merofic plus 1gm meropenem injection
- Tretiheal 0.05% Tretinoin Cream USP
- Tigebax Tigecycline 50 Mg Injection
- Bleopar-15 (Bleomycin Sulphate for Injection Bp 15 Iu)
- FETROJA CEFIDEROCOL INJECTION
- Canmab 440 mg injection
- Kolistimeth 3000000IU Injection
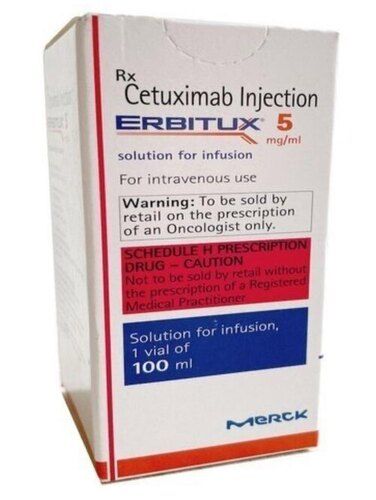
- Erbitux 5 (Cetuximab Injection)
- Pamorelin LA 22.5mg Injection (Triptorelin (22.5mg))
- Tubervac (Bacillus Calmette-Guerin Vaccine I.P)
- Abbott Tirofiban Hydrochloride Injection 5mg/100ml
- 4 - Epeedo - 50mg (epirubicin hydrochloride for injection)
- opdivo 100 mg
- daratumumab concentrate for solution for infusion 400mg
- Luprodex Depot 11.25mg (leuprolide Acetate Injection 11.25mg)
- Fluconal Fluconazole Injection(Fluconal IV)
- atezolizumab injection
- Eptifibatide 20mg/10ml Vial
- Yondelis 1 Mg Injection
- SODIUM NITROPRUSSIDE INJECTION IP
- rituximab injection 100 mg
- Capnea Injection (cipla)
- 1 MG Arsenic Trioxide Injection
- Xylistin Forte 2 Miu
- 100 MG Bevacizumab Injection
- Gufisome 50 mg injection Box Prescription
- Tigecycline 50 Mg Injection
- Eutrig HP 10000 IU Injection (chorionic gonadotrophin injection ip )
- Eptifibatide 75mg/100ml
- victoza injection
- Yervoy 50 Mg 10 Ml
- fluorouracil injection
- Novomix 30 Flexpen Biphasic Insulin Aspart
- AMBILON 50 MG
- OZEMPIC 1MG (SEMAGLUTIDE)
- PROSTAVER (ALPROSTADIL INJECTION IP 500MCG/ML)
- 100 Mg Azacitidine For Injection
- OPRESSIN (VASOPRESSIN INJECTIONS IP 20 UNITS/ML)
- Bendamiustine Hydrochloride For Injection
- TERTISSIN (TERLIPRESSIN INJ 0.1MG/ML)
- ACTILYSE 50MG
- NEOHMC (MENOTROPINFOR INJ IP )
- 50 Mg Micafungin Sodium For Injection
- ONCO BCG Bacillus Calmette-Guerin strain (40mg)
- 50 MG Oxaliplatin For USP Injection
- Reditux RA Injection Rituximab (500mg)
- Polymyxin-B Sulphate USP Injection
- GAVIR 500 (GANCICLOVIR INJ IP)
- RUTINIB CREAM ( Ruxolitinib)
- LEVEPSY (LEVETIRACETAM INJ USP 500MG/5ML )
- Fetroja cefiderocol for injection
- VICTOZA (LIRAGLUTIDE)
- 20 mg Capnea Injection
- Rabies Human Vaccine I.P
- 20 Percent Human Albumin Injection
- Bleomycin Injection
- Levosimendan For 12.5 mg Lyophilized Injection
- L-Asparaginase For Injection 10000 1.U
- Highly Purified Chorionic Gonadotropin Injection 5000 IU
- 450 MG Carboplatin Injection IP
- 3.75 Mg Leuprolide Acetate Injection
- 1 gm Meropenem Injection I.P
- Amiodarone Sterile Concentrate
- 500 mg Aciclovir Intravenous Infusion IP
- 11.25 mg Triptorelin Powder For Injection
- 420 mg Pertuzumab Injection
- 200 mg Propofl Injection IP
- Bacillus Calmette Guerin Vaccine I.P
- 5 gm Human Normal Immunoglobulin IP
- Vasopressin Injection I.P
- HUCOG 10000 HP INJECTION
- 1mg Arsenic Trioxide Pharmaceutical Injection
- 400mg Bevacizumab
- Somatropin For Injection IP
- Highly Purified Chorionic Gonadotrophin Injection
- Bictegravir 50mg Emtricitabine 200mg And Tenofovir Alafenamide 25mg Tablets
- 300mg Tenofovir Disoproxil Fumarate Tablets IP
- Piperacillin Tazobactam pybactum 4.5 mg
- Luprodex Depot 3.75mg (Leuprolide acetate for injection)
- Celodate 20mg Etomidate Injection
- Arsenic Trioxide Injection
- OCTRIDE OCTREOTIDE 100
- Phosome 50 Mg Injection
- Meroplan 1000mg Meropenem Injection
- Alburel-OS Solution For Infusion Albumin (20%)
- Bleocip Bleomycin Injection
- LMWX 60 (ENOXAPARIN SODIUM INJ IP)
- Cresp 40 Mcg
- Abhayrab Vaccine
- Xolair 150Mg (Omalizumab)
- Trodelvy 200Mg (Sacituzumab govitekan)
- Inonza 1Mg (Inotuzumab ozogamicin powder for concentrate for solution for infusion)
- Rybrevant 350Mg ( Amivantamab Liquid Concentrate for infusion 350mg)
- Eylea 40mg/ml
- Ustekirel 45mg
- Flubin 50mg (Fludarabine Phosphate for Injection USP 50mg )
- Cancyte 200mg (Gemcitabine Injection IP)
- Carmendan (Levosimendan for Injection 12.5Mg Lyophilized)
- MDS 100 (Azacitidine For Injection IP)
- Micristin 1 (Vincristine Sulphate Injection USp 1mg)
- Flubin 50mg
- Ipravent (Respirator solution )
- Ve-lcade 1 mg Injection (Bort-ezomib 1 mg)
- 3.5 mg Injection (Bortezomib 3.5 mg)
- Zometa RTU 4 mg Injection(Zoledronic acid 4 mg)
- 5mg/ml(Paclitaxel 100MG/20ML)
- Kynteles(Vedolizumab 300mg)
- SOLIRIS 300MG(Eclulizimab 300 mg / 30 ml)
- OMALIREL 150MG(Omariel (Omazilumab 150mg))
- ERBITUX(Cetuximabe 100Mg)
- SYNAGIS 50MG/0.5ML(PALIVIZUMAB)
- ULTOMIRIS 300MG/30ML(RAVULIZUMAB )
- JEMPERLI 500MG/10ML(DOSTARLIMAB INJECTION)
- QARZIBA 4.5MG/ML(DINUTUKSYMAB BETA 20MG/4.5ML)
- Darzalex Daratumumab concentrate for solution for infusion 100 mg)
- Sandostatin 0.1 mg Injection (Octreotide 0.10 mg)
- Sandostatin LAR 30 mg Injection(Octreotide 30 mg)
- Dengue IgG/IgM WB (Bioline Dengue IgG/IgM WB)
- Ferinject 1000 Mg Injection
- Crysvita 30mg/ml (Burosumab Injection)
- Rojuzda 75mg (Iuspatercept 75mg) Powder for solution for injection
- BEVAC (Hepatitis B Vaccine (rDNA) I.P
- De-fitelio 200mg Solution (Defib-rotide 200mg/2.5ml)
- Kisunal (Donanemab-azbt) 350mg/20ml
- Nabtoxol 100mg (Paclitaxel for Inj Suspension 100mg)(Albumin -Bound Nanoparticle)
- Typbar -PFS (Typhoid Polysaccharide Vaccine IP)
- Skyrizi 150Mg (Risankizumab)
- Tetanus vaccine (Adsorbed ) I.P
- Lumigan (Bimatoprodt Ophthalmic solution 0.03%)
- Equirab 1500 IU/5ml (Rabies Antiserum I.P-Equine
- Erly Prefilled Pen(Liraglutide (6mg/ml)
- Lirafit 6mg pen( Liraglutide 6mg/ml solution for inj (in prefilled pen)(rDNA Origin )
- Infubion (cyanocobalamine inj)
- Pharmaceutical Tablets
- 500 mg Sulfasalazine Gastro-Resistant Tablets IP
- Lignocaine Lox 2 Jelly
- Beloc-10 Mg (Propranolol Tablets)
- Rybelsus 7mg Semaglutide Tablets
- GABASIGN-800 Gabapentin tablets ip 800 mg
- Rybelsus Semaglutide Tablets
- Sorafenib 200 Mg Tablets
- Anastrozole TAblets IP
- Trastuzumab Emtansine Zydus Cadila Ujvira 100mg
- Gefitnat Tablets Gefitinib
- Viraday Tablets (Cipla)
- zaltrap 100 mg
- Zaltrap 100 Mg
- Xbira Abiraterone Acetate Tablets 250 Mgm(cipla)
- Fungicyte Capsules (Fluconazole Capsules IP 150mg)
- Mycophenolic Acid Tablet usp 360 mg
- Zidolam N Zidovudine Lamivudine Nevirapine Tablets
- Ivosenib 250mg
- Gabapentin tablets ip 800 mg
- trastuzumab emtansine 100 mg
- Xtane Exemestane Tablets
- Anastrozole Tablets IP
- 8 mg Dexamethasone IP Tablets
- 500 mg Azithromycin IP Tablets
- CYTOFLU Flucytosine Tablets IP
- cetuximab erbitux
- Semaglutide Tablets 14mg
- Acetate Tablets
- Trima 150mg Tablet (Moclobemide Tablets 150 Mg)
- 1 mg Anastrozole IP Tablets
- Dexamethasone Tablets IP
- Mycofit (Mycophenolic Acid Tablet usp 360 mg)
- Rosuvastatin Tablet 10mg (Rosubest 10)
- trodelvy 200 mg
- Imat jala 400mg
- PIRFENEX 200 MG (Pirfenidone)
- ALKACEL 2MG (MELPHALAN )
- SORAFENAT (SORAFENIB TOSYLATE )
- Dexamethasone IP Tablets
- 400 mg Sofosbuvir Tablets
- 6-MP (MERCAPTOPURINE )
- Methimazole USP Tablet
- Sofosbuvir Tablets 400 mg
- YERVOY 50
- Rifaximin Tablets
- VELASOF (VELPATASVIR SOFOBUVIR )
- Sofosbuvir Tablet
- 150 mg Moclobemide Tablets
- 12 mg Ivermectin Tablets
- Alpha Ketoanalogues Of Essential Amino Acids Tablets
- 20 mg Tamoxifen Tablets IP
- Sodium Chloride Tablets
- 10 mg Propranolol Tablets
- Deferasirox Tablets
- Etoricoxib And Thiocolchicoside Tablets
- 360 mg Mycophenolic Acid Delayed Release Tablets USP
- 6 mg Dapoxetine Hydrochloride Tablets
- 200 mg Sorafenib Tablets IP
- Tenofovir Disoproxil Fumarate 300mg Efavienz 600mg And Emtricitabine Tablets IP 200mg
- 200 Mg Emtricitabine And 300 Mg Tenofovir Disoproxil Fumarate Tablets
- 300 Mg Atazanavir And Ritonavir Tablets
- 200 Mg Voriconazole Tablets
- Exemestane Tablets IP
- Myfocept-S 360
- Rifagut 550 Tablet (Rifaximin (550mg))
- Aplazar Tablet (Alpha Ketoanalogue (200mg))
- Tenolame Tablets
- Ledifos tablets
- Daruvir 600 R Tablet (Darunavir And Ritonavir Tablets)
- Vonaday tablets
- Tamodex 20 Mg Tablet
- DUOVIR TABLET
- Modafresh 200 Tablets
- DECMAX (Dexamethasone)
- tenofovir disoproxil fumarte
- Vonavir Tab (emcure)
- Tencfovir Disoprocil Fumarate Lamivudine Efavirenz Tables Ip(Emcure)
- Imatib Tablets IP 400mg (Imatib 400)
- Hepcvir Sofosbuvir 400 Mg Tablets (cipla)
- Tafero Tenofovir Alafenamide Tablets
- Desirox 500 Tablet (cipla)
- Poxet 60 Mg Tabs (Dopoxetine HCI Tablets 60mg)
- Duovir (Lamivudine And Zidovudine Tablets Ip)
- Tastylia 20 Mg Strips
- 7mg Semaglutide Tablets
- Soranib (Sorafenib 200 Mg Tablets)
- Sevcar -800Mg (Sevelamer carbonate Tablets 800Mg)
- Abiracan (Abiraterone Acetate Tablets IP 250mg)
- Prevymis 240Mg (Letermovir)
- Casodex 50 mg Tablet(Bicalutamide 50 mg)
- Tauritmo 25 mg Capsule(Midostaurin 25 mg)
- Asunra 400 mg Tablet(Deferasirox 400 mg)
- Ramiven 150mg Tablet(Abemaciclib)
- Xospata 40mg Tablet(Gilteritinib )
- INLYTA 5MG(Axitinib Tablets)
- Proglicem 25(Diazossido 25mg)
- OSIMERT(Osimetirib 80mg)
- NUBEQA(Darolutamida 300mg (NUBEQA))
- MEQSEL 2MG(Trametinibe 2mg)
- Tafinlar 75mg(Dabarafenib)
- RYBELSUS 3MG(SEMAGLUTIDE TABLETS)
- LYSODREN 500MG(MITOTANE TABLETS USP)
- CAPRELSA 300MG(VANDETANIBUM )
- Afinitor 10 mg Tablet (Everolimus 10 mg)
- Afinitor 5 mg Tablet (Everolimus 5 mg)
- Certican 0.25 mg Tablet (Everolimus 0.25 mg)
- Certican 0.5 mg Tablet(Everolimus 0.5 mg)
- Certican 0.75 mg Tablet (Everolimus 0.75 mg)
- Fem-ara 2.5 mg Tablet(Letr-ozole 2.5 mg)
- Glivec 100 mg Tablet (Imatinib 100 mg)
- Meqsel 0.5 mg Tablet(Trametinib 0.5 mg)
- Nolvadex 10 mg Tablet(Tamoxifen 10 mg)
- Tagrisso 80 mg Tablet (Osimertinib 80 mg)
- Caprelsa 300mg (Vandetanib ) tab
- Revolade 50mg (Eltrombopag Tab)
- Lucivand 300mg (Vandetanib Tab)
- Jakavi 15mg Tab (Ruxolitinib)
- Rebopag 25mg (Eltrombopag Tab 25mg )
- Premarin (Conjugated Estrogens Tablet U.S.P)
- Zelboraf 240mg Tab (Vemurafenib)
- Rinvoq 15mg Tab (updacitinib)
- Paxobrook Tab (Nirmatrelvir tablet 150mg & Ritonavir Tab Ip 100mg Combipack )
- Strepsils (Medicated Throat Lozenges For Sore Throat Flavor Orange)
- Pure Nutrition Multivitamin ( for Women with Minerals For Healthy Bones & Immunity Tablet)
- Pure Nutrition Vitamin D3+K2 Tab (for Immunity, Bones & Heart Health Veg Tablet)
- Pure Nutrition Vitamin B12 Tab 1500mcg (From Methylcobalamin Supports Nerve Health)
- Maharishi Ayurveda (Amlant Acid -Balance Management)
- Pharmaceutical Capsules
- Lenalid 10 Mg Lenalidomide
- 4 MG Lenvatinib Capsules
- 250 mg Mycophenolate Mofetil Capsules
- 40 mg Enzalutamide Capsules
- Vingraf Capsules TACROLIMUS
- Enzalutamide Capsule
- Nitib Ibrutinib 140 Mg Capsules
- Esomeprazole And Domperidone (SR) Capsules
- 140 mg Ibrutinib Capsules
- Avosteride 0.5 Capsules
- Temoside Temozolomide 250 Mg Capsule
- 500 mg Hydroxyurea Capsules IP
- Lenangio 10 Mg Capsule
- Prebel IP 500mg
- Cytodrox Hydroxyurea Capsules
- Ibrutinib 140 Mg Capsules
- 40 MG Enzalutamide Capsules
- 0.5 MG Tacrolimus IP Capsules
- 10 mg Lenalidomide Capsules
- 250 mg Temozolomide Capsules
- ALPHADOL 0.25 (Alfacalcidol)
- Bilypsa Tablet
- Deferiprone Capsule(Kelfer)
- Allopathic Thalidomide Capsules(Thombil)
- Prograf tacrolimus0.5mg Capsule
- Ibrance Capsules
- Mydrea(Hydroxyurea Capsules IP 500mg)
- CA Atra 10 Softgel Capsule (Tretinoin (10mg)
- Rafinlar 50 mg Capsule (Dabrafenib 50 mg)
- Erleada 60mg (Apalutamide )
- Pangraf 1.0mg (Tacrolimus Capsules IP 1.0mg)
- Mycept-S 360mg (Mycophenolic Acid Delayed-Release Tab USP 360mg)
- Vonadice 20mg (Vonoprazan Tab 20mg)
- Lucipral 100Mg( Pralsetinib capsules)
- Rutinib Cream (Ruxolitinib)
- Hamdard (Itrifal Ustukhuddus)
- Niranib 100mg Cap (Niraparib Cap)
- Pure Nutrition Testoboost Tab (Sexual Wellbeing Veg Tablet)
- Pharmaceutical Syrup
- Asthalin Inhaler
- Mouthwash
- Pharmaceutical Injection
- Contact Us
Showroom
Introducing our innovative pharmaceutical injection, a cutting-edge solution for targeted and rapid medical treatment. Formulated with precision, this injectable medication ensures swift absorption and reliable therapeutic outcomes. Trust in our commitment to quality and efficacy as we strive to elevate healthcare standards with this advanced pharmaceutical injection.
Experience optimal health with our pharmaceutical tablets, meticulously crafted for superior efficacy. These tablets offer a convenient and precise dosage, delivering rapid relief and sustained wellness. Trust in our commitment to quality and innovation as we prioritize your well-being with our advanced pharmaceutical tablet formulation.
Unlock health and vitality with our pharmaceutical capsules, a sophisticated blend of precision and efficacy. These capsules are crafted to deliver targeted relief and sustained wellness. Trust in our commitment to quality and innovation as we prioritize your well-being with our advanced pharmaceutical capsule formulation.
Revitalize your health with our pharmaceutical syrup, a carefully formulated solution for comprehensive wellness. This syrup combines efficacy with a palatable taste, ensuring easy consumption for all ages. Trust in our commitment to quality and innovation as we prioritize your well-being with our advanced pharmaceutical syrup formulation.
Relieve respiratory distress swiftly with Asthalin Inhaler. A trusted bronchodilator, it offers rapid relief from asthma symptoms. Compact and easy to use, this inhaler ensures precise dosage for on-the-go respiratory support. Trust in its proven effectiveness, providing quick and targeted relief for those in need of respiratory assistance.
Elevate your oral care routine with our refreshing Mouthwash. Specially formulated for lasting freshness, it combats plaque and bacteria, promoting healthy gums and minty-fresh breath. Rinse away the day with this alcohol-free solution, ensuring a confident and clean smile. Prioritize your oral health with our effective and invigorating Mouthwash.

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese